Just quickly ... @MidwesternDoc is a Midwestern Doc
On X, @RobertKennedyJr, known for his lavish scepticism, reposted the following thread.
MidwesternDoc has a substack titled Forgotten Side of Medicine which the Doc says has 109k subscribers.
Initially I sniped at him (above); it feels sufficient, and who can find any time to evaluate the kooks’ output?
But now I’m looking for a distraction, and I recall the Doc. I glance at the post he’s promoting in the X thread: The Great Ozempic Scam and The Safe Ways to Lose Weight.
Spurious claims are made with sloppy language and left hanging, e.g. the FDA pushes Ozempic and Rob Califf (head of the FDA) has ‘immense conflicts of interest’. But I am sifting thru these words, hunting for a comment, this:
One thing I’ve been immensely curious about is what life is actually like behind the scenes within the pharmaceutical industry.
Okay: the Doc is a doc; he’s uninitiated, he’s ignorant - of course. And apparently he would like to hear from us (insiders). There is something else to bear in mind: within the drug industry docs are widely known to be feeble (due to their ignorance) and easily cajoled.
A case in point: the Doc is inclined to mention the opioid scandal. How could the scandal have occurred without the willing cooperation of the docs? Bad Pharma. Bad Docs? We should not wait for Goldacre to pen that book.
After confirming the Doc’s ignorance, I wanted to find something legitimate, a specific claim that can be confronted head-on. Thus: jump to the section titled The Risks and Benefits of Ozempic.
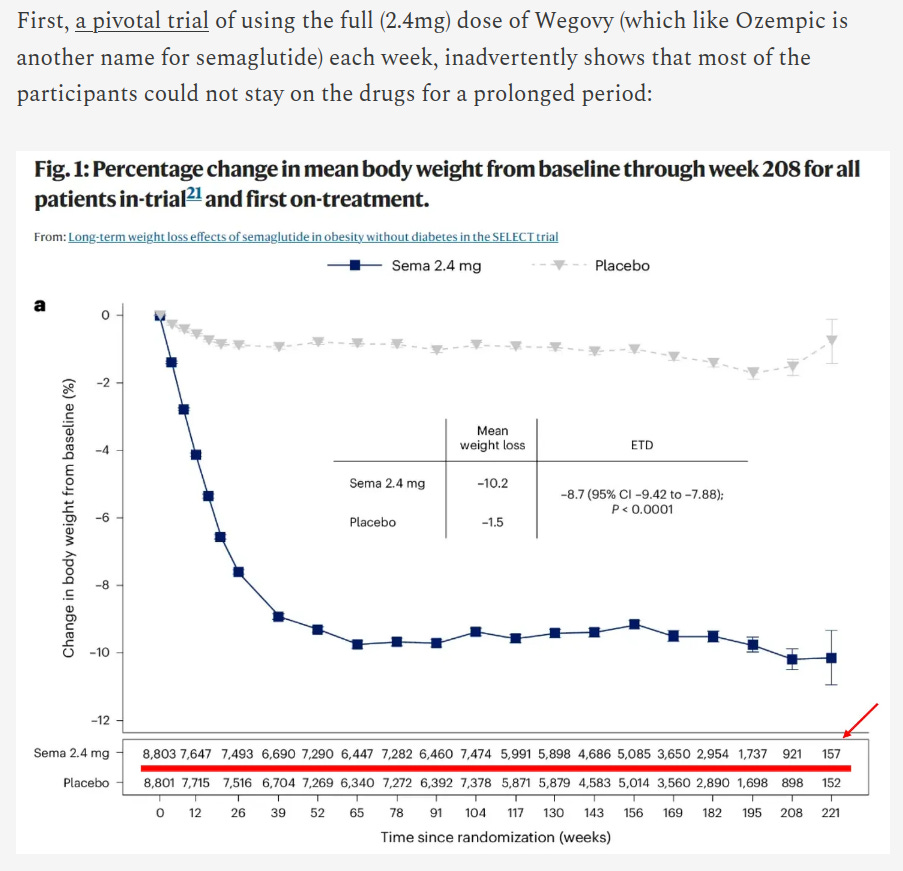
Referring to the pivotal clinical trial of semaglutide, the Doc declares: “Most of the participants could not stay on the drugs for a prolonged period.”
I can point out that the Doc has simply misunderstood this plot, but it’s more important to understand what the error says about the Doc, than the error itself.
This trial, the SELECT trial, had an interim analysis; patients are recruited in a staggered fashion; then the interim analysis occurs at the pre-specified time according to the accrual of events; the interim analysis determines that the study should be terminated (according to the stopping rule inherent in the study design).
This explains why the number of patients is trailing off: the follow-up is variable, it has nothing to do with compliance.
There is a lot more that could be said about compliance, estimands, the complexity of multiple imputation methods for handling missing data, mixed modelling for repeated measures, etc., but none of it is relevant to the point I wish to make.
The point is that the Doc does not understand trial design and he is telling his readers, one hundred and nine thousand of them, a falsehood. More to the point: the detail of the study design is freely available on clinicaltrials.gov, and the drop-out rate he implies would not be seen in a large quality trial like this (anyone with any time in drug development would know that).
The Doc continues ... He finds it unreasonable that the weight loss attributable to the drug is partially regained when the patient is taken off drug - figure below. But it is a lot to expect of a drug, no? I.e. that it continues to work when you are no longer taking it. And if the Doc were a good cynic, rather than a mediocre one, he would ponder how the company might use this result in their marketing materials.
The Doc then moves to safety and misleads the reader in a way that is incredibly hack. He displays the adverse events reported in the trial for the drug and not for the control (placebo). It is somehow more dishonest when the distortion lacks originality. E.g., we can see that 8% of those on the active drug had serious adverse events! (For the placebo group it was 12%.)
The Doc is inciting distrust and disguises his incentives by using ‘Bad Pharma’ as a counterpoint: he’s alerting us to the misdeeds of greedy Pharma.
If the reader remembers one thing they should remember that the Doc confessed he’s an outsider; we can infer from this that he has never met Califf or collaborated with him, although he insinuates that he’s corrupt; and he has never been privy to regulatory interactions or designed a trial etc.; i.e. he’s guessing.
If there were time we would like to comment more on safety. (People may not realise GLP-1 agonists have been around for many years and there have been ongoing safety discussions with the regulatory authorities.) Maybe another time.
It would be convenient to make brief posts like this one, at least it seems doable. We will title them ‘Just quickly ...’